Study on Liquid Structure and Dynamics by X-ray diffraction and neutron scattering
Water conforms to the shape of the vessel that contains it. In a liquid state, liquid molecules move freely, hence the structure of liquids is characterized by the absence of long-range order which defines crystalline materials. However, liquids have a short and middle range order originated from molecular interactions. Liquids play a fundamental role in the chemical, engineering, and biological processes. To clarified underling mechanism of functionality expression of liquids, I investigate structure and dynamics of liquid by X-ray and neutron scatterings. My research themes are as follows:
I. Dynamics of Liquids investigated by neutron scattering
In the dynamics study of liquids, computer simulation assuming intermolecular potential is often used. However, determination of intermolecular potential is a weak point of the physical property evaluation of liquids. Neutron/X-ray scattering are possible to directly observe dynamic structural factors and intermediate scattering functions of liquid. Physical properties of liquids were evaluated from these functions, and the results were compared with experimental values. In addition, this research can also verify the intermolecular potential used for computer simulation. The dynamics of liquid was clarified experimentally such as benzene and methanol, dynamics of nanoparticle dispersion solution, electrolyte solution of lithium-ion battery and so on.[J. Phys. Chem. B in press, Chem. Phys. Lett. 680, 1-5 (2017), J. Phys. Chem. B, 121 (21), 5355-5362 (2017), J. Mol. Liquids 222, 395-397 (2016)]
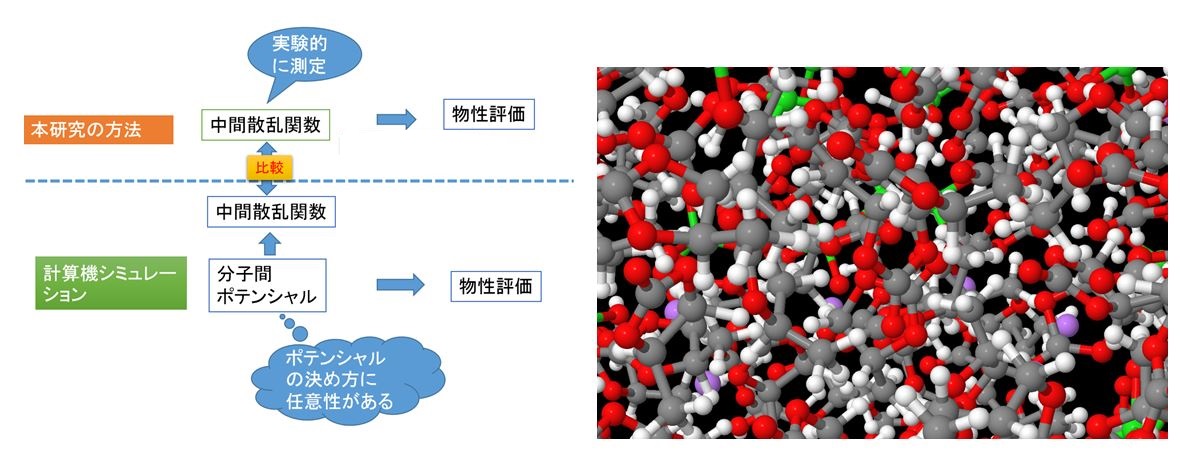 Fig. 1. Solution structure of electrolyte solution of lithium-ion battery
Fig. 1. Solution structure of electrolyte solution of lithium-ion battery
II.Structure and dynamics of confined water
Water confined in the nanoscale pores is stable at supercooled state, which is quite different from the bulk, and attracts attention as a model of unfrozen water in the living body. The water adsorbed on MCM - 41 which is one of the porous silica glasses with uniform pore size was measured by neutron spin echo method. It is found that the dynamics of the water molecules in the pores is slower than that of the bulk and that there is a distribution in the relaxation time. The temperature dependence of the relaxation time of the confined water shows the fragile-to-strong crossover around 230 K. It might be relevant to the second critical point of water which explains the Anomalous properties of water, such as maximum density of water at 4°C. [Pure Appl. Chem. 85, 289-305 (2013), Bunsekikagaku, 61 (12), 989-998 (2012), Anal. Sci. 28, 639-41 (2012), J. Phys: Condens. Matter 24, (2012) 064101, J. Chem. Phys. 129, 054702 [1-11] (2008) ]
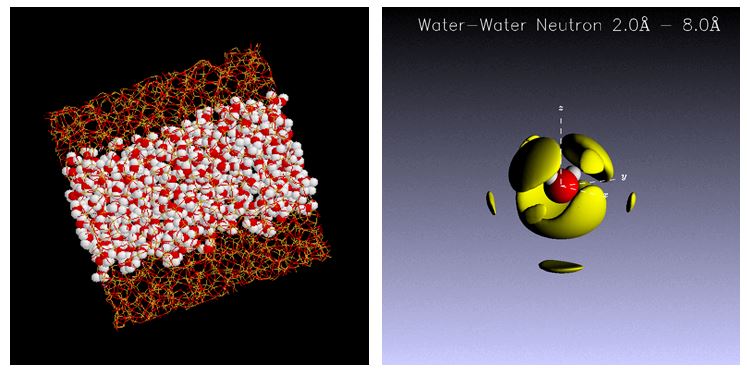 Fig. 2. Water confined in meso porous silica
Fig. 2. Water confined in meso porous silica
III.Microscopic structure of alcohol-water mixture and structure change of protein in the mixture
Alcohol and water are mixed homogeneously. However, at a molecular level, water and alcohol clusters are formed and a nano-heterogeneous structure appears. The heterogeneous structure would affect various chemical processes. In this study, I focus on the structural change of proteins in alcohol-water mixture. The denaturation mechanism of protein induced by alcohol was revealed by small angle X-ray and neutron and molecular dynamics calculation.
[J. Mol. Liquids 189, 1-8 (2014), Z. Naturforsch. 68a, 145-151 (2012), BBA - Proteins and Proteomics 1824, 502-510 (2012), Phys. Chem. Chem. Phys. 12, 3260-3269 (2010), Pure Appl. Chem. 80, 1337-1347, (2008), Fukuoka University Science Reports, 37(1), 23-31 (2007), Chem. Phys. Lett. 412, 280-284 (2005)]
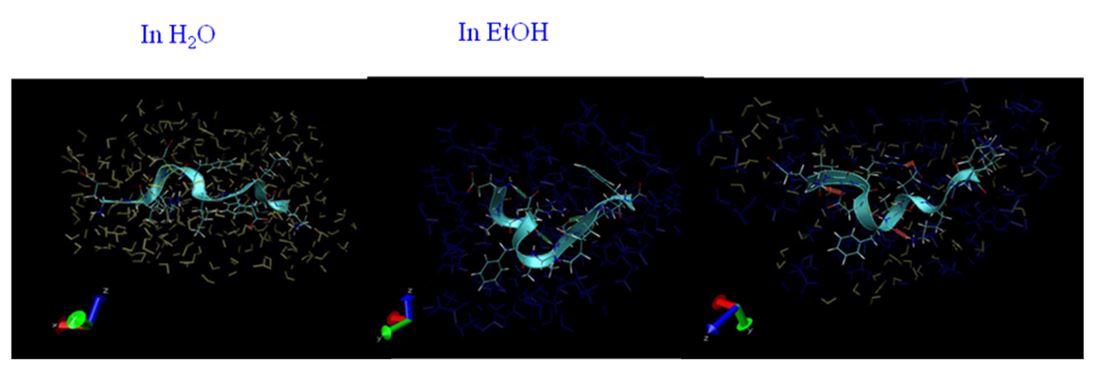 Fig. 3. Hydration structure of 10 residue peptide in water and ehtanol
Fig. 3. Hydration structure of 10 residue peptide in water and ehtanol
IV. Cluster structure of supercritical water and methanol
A supercritical fluid is any substance at a temperature and pressure above its critical point. In the supercritical state, phase transition of liquid and gas is not observed even if the pressure is changed. Hydrogen bonding liquid such as water and alcohol, the critical temperature and pressure are generally high. Small-angle neutron scattering has been measured for supercritical water, the intermolecular interaction of the fluids remains, indicating that it is related with unique properties as seen in the chemical reaction in the supercritical fluid. In addition, X-ray inelastic scattering measurement of supercritical water investigated the cluster formation and disruption in a subfemto second time scale. [Chem. Phys. Lett. 440, 210-214 (2007), J. Phys. Chem. Sol. 66, 2246-2249 (2005)]
Contact
9号館3階 Room 9321 Phone 6241
E-mail: kyoshida[at]fukuoka-u.ac.jp
